Appropriate use of antibiotics
The overuse of antibiotics is widely accepted as a major driver of some emerging infections (such as Clostridium difficile) and for the continued development of antimicrobial resistance (AMR). The growing emergence of multi-drug resistant organisms and the limited development of new agents available to counteract them have caused an impending public health crisis of international concern, threatening modern medicine, animal health and food security.
AMR is a natural phenomenon that occurs as microbes evolve. However, human activities have accelerated the pace at which microorganisms develop and disseminate resistance. Inappropriate use of antibiotics and other antimicrobials, as well as poor prevention and control of infections, are contributing to the development of AMR.
Appropriate use of antimicrobials is an integral part of good clinical practice. Clinicians should be aware of their role and responsibility for maintaining the effectiveness of current and future antibiotics.
Antimicrobial resistance
AMR is one of the greatest threats to public health, sustainable development and security worldwide. Its prevalence has increased alarmingly over the past decades.
Infections caused by antibiotic-resistant bacteria continue to be a challenge. Rice in 2008 coined the acronym of “ESKAPE” pathogens including Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species to emphasize that these bacteria currently cause the majority of hospital infections and effectively “escape” the effects of antibacterial drugs. These organisms are increasingly multi-drug- (MDR), extensive-drug- (XDR) and pan-drug- resistant (PDR) and this process is accelerating globally.
The burden of AMR is difficult to quantify in some regions of the world because enhanced surveillance requires personnel, equipment and financial resources that are not always available. However, the worldwide impact of AMR is significant in terms of economic and patient outcomes.
The global nature of AMR calls for a global response, both in the geographic sense and across all involved healthcare sectors. The impact of AMR worldwide is significant, both in economic terms and patient outcomes, as it may:
- Lead to some infections becoming untreatable or requiring antimicrobials of last resort when the treatment is mandatory
- Increase length of hospital stay, morbidity, mortality and treatment cost.
The World Health Organization (WHO) endorsed a global action plan to tackle antimicrobial resistance. It sets out five strategic objectives:
- To improve awareness and understanding of antimicrobial resistance
- To strengthen knowledge through surveillance and research
- To reduce the incidence of infection
- To optimize the use of antimicrobial agents
- To develop the economic case for sustainable investment that takes account of the needs of all countries, and increase investment in new medicines, diagnostic tools, vaccines and other interventions.
Although most physicians are aware of the problem of antimicrobial resistance, most underestimate this problem in their own hospital and prescribe inappropriately antibiotics. They can help tackle resistance by:
- Enhancing infection prevention and control
- Controlling the source of infection when it is needed
- Prescribig antibiotics only when they are truly required
- Prescribing appropriate antibiotic(s) with adequate dosages to treat the infections
- Using the shortest duration of antibiotics based on evidence of guidelines
- Educating the communities in which we work of the need to use antibiotics wisely.

Antibiotics use: finding the right balance
The substantial problem of AMR is especially relevant to antibiotic resistance (ABR), although antifungal resistance is increasing at an alarming rate. Although the phenomenon of ABR can be attributed to many factors, there is a well-established relationship between antibiotic prescribing practices and the emergence of antibiotic resistant pathogens.
Clinicians should always optimize antibiotic management to maximise clinical outcome and minimize emergence of the development of resistance and the selection of resistant pathogens. The necessity of formalized systematic approaches to the optimization of antibiotic therapy in the setting of surgical general surgery units worldwide, both for elective and emergency admissions, has become increasingly urgent.

Antibiotic prophylaxis
Systemic antibiotic prophylaxis (AP) is one of the most important component of a perioperative infection prevention strategy. The use of AP contributes considerably to the total amount of antibiotics used in hospitals and may be associated with increases in antibiotic resistance and healthcare costs.
Although AP plays a pivotal role in reducing the rate of surgical site infections, other factors such as attention to basic infection-control strategies may have a strong impact on surgical site infections rates.
Perioperative surgical AP should be recommended for operative procedures that have a high rate of postoperative wound infection or when foreign material is implanted.
Prophylactic antibiotic agents should be nontoxic and inexpensive and have in vitro activity against the common organisms that cause postoperative wound infection after a specific surgical procedure.
Therapeutic concentrations of antibiotics should be present in the tissue throughout the all period that the wound is open.

Antibiotic therapy
Antibiotics should be used after a treatable infection has been recognized or when there is a high degree of suspicion for infection. Initial antimicrobial therapy is typically empirical in nature because they need immediate treatment (especially in critically-ill patients), and microbiological data (culture and susceptibility results) usually requires ≥24 h for the identification of pathogens and antibiotic susceptibility patterns
The decision tree for the empiric antibiotic regimen should depend mainly on three factors: presumed pathogens involved and risk factors for major resistance patterns, clinical patient’s severity and presumed/identified source of infection.
Knowledge of local rates of resistance and the risk factors that suggest resistant bacteria should be involved as essential components of the clinical decision-making process when deciding on which antibiotic regimen to use for empiric treatment of infection
The timing, regimen, dose and duration of antimicrobial therapy should be always optimised.
Antimicrobial therapy should be shortened for patients demonstrating a positive response to treatment.


Dosage
The antibiotic dosing regimen should be established depending on host factors and properties of antibiotic agents. Antibiotic pharmacokinetics describes the fundamental processes of absorption, distribution, metabolism, and elimination and the resulting concentration-versus-time profile of an agent administered in vivo. The achievement of appropriate target site concentrations of antibiotics is essential to eradicate the relevant pathogen. Suboptimal target site concentrations may have important clinical implications, and may explain therapeutic failures, in particular, for bacteria for which in vitro MICs are high. Antibiotics typically need to reach a site of action outside the plasma. This requires the drug to pass through the capillary membranes. Disease and drug-related factors can contribute to differential tissue distribution.
Knowledge of the pharmacokinetic and pharmacodynamic antibiotic properties of each drug including (inhibition of growth, rate and extent of bactericidal action, and post-antibiotic effect) may provide a more rational determination of optimal dosing regimens in terms of the dose and the dosing interval. Optimal use of the pharmacokinetic/pharmacodynamic relationship of anti-infective agents is important for obtaining good clinical outcomes and reduction of resistance. Dosing frequency is related to the concept of time-dependent versus concentration-dependent killing. Beta-lactams exhibit time-dependent activity and exert optimal bactericidal activity when drug concentrations are maintained above the MIC. Therefore, it is important that the serum concentration exceeds the MIC for appropriate duration of the dosing interval for the antimicrobial and the organism. Higher frequency dosing, prolonged infusions and continuous infusions have been utilized to achieve this effect. In contrast, antibiotics such as aminoglycosides exhibit concentration-dependent activity and should be administered in a once daily manner (or with the least possible number of daily administrations) in order to achieve high peak plasma concentrations. With these agents, the peak serum concentration, and not the time the concentration remains above the MIC, is more closely associated with efficacy. In terms of toxicity, aminoglycosides nephrotoxicity is caused by a direct effect on the renal cortex and its uptake saturation. Thus, an extended interval dosing strategy reduces the renal cortex exposure to aminoglycosides and reduces the risk of nephrotoxicity.
In patients with septic shock, administering an optimal first dose is probably as equally important as to the timing of administration. This optimal first dose could be described as a loading, or front-loaded dose and is calculated from the volume of distribution (Vd) of the drug and the desired plasma concentration. The Vd of hydrophilic agents (which disperse mainly in water such as beta-lactams, aminoglycosides and glycopeptides) in patients with septic shock may be altered by changes in the permeability of the microvascular endothelium and consequent alterations in extracellular body water. This may lead to lower than expected plasma concentrations during the first day of therapy resulting in sub-optimal achievement of antibiotic levels. In the setting of alterations in the volume of distribution, loading doses and/or a higher overall total daily dose of beta-lactams, aminoglycosides, or glycopeptides are often required to maximize the pharmodynamics ensuring optimal drug exposure to the infection site in patients with sepsis or septic shock.
Once an appropriate initial loading dose is achieved, the antibiotic regimen should be reassessed, at least daily, because pathophysiological changes may significantly affect drug availability in the critically ill patients. Lower than standard dosages of renally excreted drugs must be administered in the presence of impaired renal function, while higher than standard dosages of renally excreted drugs may be needed for optimal activity in patients with glomerular hyperfiltration. It should be noted that in critically ill patients, plasma creatinine is an unreliable marker of renal function.
Duration
Duration of therapy should be shortened as much as possible unless there are special circumstances that require prolonging antimicrobial therapy such as immunosuppression, or ongoing infections. Oral antimicrobials, can substitute IV agents as soon as the patient is tolerating an oral diet so as to minimize the adverse effects which are associated with intravenous access devices. Where possible, conversion to oral antimicrobial agents having high oral bioavailability (e.g. fluoroquinolones) should be considered. Patients with intra-abdominal infections who have signs of sepsis beyond 5 to 7 days of treatment warrant aggressive diagnostic investigation to determine if an ongoing uncontrolled source of infection or antimicrobial treatment failure is present. In the management of critically ill patients with sepsis and septic shock clinical signs and symptoms as well as inflammatory response markers such as procalcitonin, although debatable, may assist in guiding antibiotic treatment.
Antimicrobial stewardship programs
Given the urgent need to improve antimicrobial use in healthcare all acute care hospitals should implement Antibiotic Stewardship Programs (ASPs). ASP is an emerging strategy designed to optimize outcomes and reduce the emergence of resistant organisms. Hospital based ASPs can help clinicians both to optimize the treatment of infections and reduce adverse events associated with antibiotic use.
The best strategies for an ASP are not definitively established and are likely to vary based on local culture, policy and routine clinical practice. Promotion of ASPs across clinical practice is crucial to their success to ensure standardization of antibiotic use within an institution. We propose that the best means of improving antimicrobial stewardship should involve collaboration among various specialties within a healthcare institution including prescribing physicians.
Successful ASPs should focus on collaboration between all healthcare professionals to shared knowledge and widespread diffusion of practice. Involvement of prescribing physicians in ASPs may rise their awareness on antimicrobial resistance.
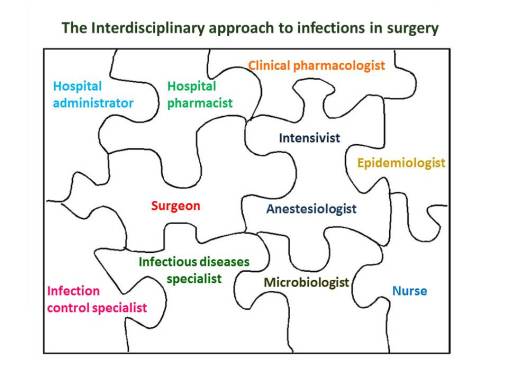
The best organization of ASPs should provide all professionals involved in both prevention and treatment of infections including epidemiologists, infection control specialists, infectious diseases specialists, microbiologists, clinical pharmacologists, hospital pharmacists, surgeons, intensivists, anesthesiologists and nurses. Only by a cohesive approach with a direct involvement of prescribing physicians the battle against the inappropriate use of antibiotics in hospitals worldwide may be won.